Phase change worksheet answers provide the steps needed to solve problems related to the change of physical state of a substance from one phase to another. They are usually found in the form of diagrams, tables, and equations. They are a great way to learn about the different changes of state, such as melting and boiling points, and how to calculate the amount of energy required for a certain change. They can also be used to demonstrate the relationship between temperature, pressure, and volume. Understanding the basics of phase change is essential for understanding many real-world applications, such as the manufacture of certain products or the operation of certain machines.
Understanding the Basics: A Comprehensive Guide to Phase Change Worksheet Answers
The purpose of this worksheet is to provide a comprehensive overview of phase change. This will include a discussion of the various types of phase changes, the factors that influence them, and the impact that they have on different materials.
1. What is a phase change?
A phase change is a physical change in the state of matter, such as melting, freezing, or vaporization. These changes are caused by the addition or removal of energy, and can affect the temperature and pressure of a given substance.
Contents
- 0.1 Understanding the Basics: A Comprehensive Guide to Phase Change Worksheet Answers
- 0.2 Exploring Different Types of Phase Changes: Examining the Various Types of Phase Change Worksheet Answers
- 0.3 Using Phase Change Worksheet Answers to Predict Chemical Reactions
- 0.4 Analyzing the Effects of Heat and Pressure on Phase Change: Examining the Impact of Heat and Pressure on Phase Change Worksheet Answers
- 0.4.1 1. Explain the general relationship between heat and pressure and the phase change process.
- 0.4.2 2. Describe how heat can affect the phase change process.
- 0.4.3 3. Describe how pressure can affect the phase change process.
- 0.4.4 4. Explain the implications of these effects on phase change in practical applications.
- 0.5 Applying Phase Change Worksheet Answers to Real-World Problems: Exploring How Phase Changes Can Be Used in Everyday Situations
- 0.6 Images of Phase Change Worksheet Answers
- 0.7 Download Phase Change Worksheet Answers
- 1 Conclusion
- 1.1 Some pictures about 'Phase Change Worksheet Answers'
- 1.1.1 phase change worksheet answers
- 1.1.2 phase change worksheet answers pdf
- 1.1.3 phase change worksheet answers grade 8
- 1.1.4 phase change worksheet answers chemistry
- 1.1.5 phase change phenomena worksheet answers
- 1.1.6 phase change graphs worksheet answers
- 1.1.7 phase change descriptions worksheet answers
- 1.1.8 phase change problems worksheet answers
- 1.1.9 phase change identification worksheet answers
- 1.1.10 phet phase change worksheet answers
- 1.2 Related posts of "Phase Change Worksheet Answers"
- 1.1 Some pictures about 'Phase Change Worksheet Answers'
2. What are the different types of phase changes?
There are four main types of phase changes: melting, freezing, condensation, and evaporation. Melting is the process of a substance changing from a solid to a liquid state. Freezing is the reverse process, where a liquid turns into a solid. Condensation is the process of a gas becoming a liquid, while evaporation is the reverse.
3. What are the factors that influence phase changes?
The factors that influence phase changes include temperature, pressure, and the properties of the material. Increasing the temperature can cause a substance to melt, while decreasing the temperature can cause it to freeze. Increasing the pressure can cause a substance to condense, while decreasing the pressure can cause it to evaporate. The physical properties of a material, such as its boiling point and melting point, can also affect its phase changes.
4. What impact do phase changes have on materials?
Phase changes can have a significant impact on materials. For example, when a substance melts, it can increase its volume and decrease its density, leading to a change in its shape and structure. Similarly, when a substance evaporates, it can decrease in volume and increase in density, leading to a change in its properties. In addition, phase changes can affect the physical and chemical properties of a material, such as its solubility, reactivity, and electrical conductivity.
Exploring Different Types of Phase Changes: Examining the Various Types of Phase Change Worksheet Answers
Phase changes are changes in a substance’s physical state due to changes in temperature or pressure. Any substance can experience a phase change; however, there are four common types of phase changes: melting, freezing, evaporation, and condensation.
Melting is a phase change in which a solid is converted to a liquid. This change occurs when the temperature of a solid increases above the melting point of the substance. For example, when ice is heated, it will melt and become liquid water.
Freezing is the reverse of melting. It is a phase change in which a liquid is converted to a solid. This change occurs when the temperature of a liquid decreases below the freezing point of the substance. For example, when liquid water is cooled, it will freeze and become ice.
Evaporation is a phase change in which a liquid is converted to a gas. This change occurs when the temperature of a liquid increases and the pressure of the liquid is decreased. For example, when water is heated, it will evaporate and become water vapor.
Condensation is the reverse of evaporation. It is a phase change in which a gas is converted to a liquid. This change occurs when the temperature of a gas decreases and the pressure of the gas is increased. For example, when water vapor is cooled, it will condense and become liquid water.
Phase changes are important to understand because they are essential for understanding the physical properties of matter. By examining the various types of phase changes, we can gain insight into how matter behaves under different conditions.
Using Phase Change Worksheet Answers to Predict Chemical Reactions
In chemistry, predicting the outcome of a reaction is an essential skill for scientists and students alike. To aid in this process, a worksheet known as a phase change worksheet can be used. This document is a valuable resource for predicting the products of a chemical reaction given the reactants.
A phase change worksheet is a form of table that lists the reactants and products of a chemical reaction. This worksheet is designed to help the user determine the products of a reaction when given the reactants. It works by breaking the reactants down into their component elements and then listing the possible product combinations. The user can then use this information to predict the product of the reaction.
The phase change worksheet can be used in a variety of different ways to predict the outcome of a reaction. It can be used to determine the products of a single reaction, or it can be used to determine the products of multiple reactions. Additionally, the worksheet can be used to determine the products of a reaction that involves multiple reactants.
When using a phase change worksheet to predict the outcome of a reaction, it is important to understand how the elements interact. This is because the reactants and products can be determined from the elements and their interactions. As such, it is essential to understand the basics of chemical reactions and the elements involved.
In addition to predicting the products of a reaction, the phase change worksheet can also be used to determine how the reactants interact to form the products. This can be done by looking at the elements in the worksheet and determining how they interact to form the products. By understanding the interactions between the elements, the user can determine the products of a reaction.
Overall, the phase change worksheet is a valuable tool for predicting the products of a chemical reaction. By understanding the reactants and products of a reaction, the user can use the worksheet to accurately predict the outcome of a reaction. With this knowledge, the user can confidently determine the products of a reaction and plan accordingly.
Analyzing the Effects of Heat and Pressure on Phase Change: Examining the Impact of Heat and Pressure on Phase Change Worksheet Answers
Phase change is a fundamental process in which matter changes from one physical state to another. Heat and pressure are two factors that play a major role in this process, and their effects on phase change have been studied extensively. This worksheet explores the impact of heat and pressure on phase change.
1. Explain the general relationship between heat and pressure and the phase change process.
Heat and pressure both play a significant role in the process of phase change. Heat increases the kinetic energy of particles, which can cause a substance to move from a solid to a liquid or from a liquid to a gas. Similarly, pressure can also force particles to change their state as it can push them closer together or further apart. The increased pressure causes the particles to vibrate, which can cause them to enter a different phase.
2. Describe how heat can affect the phase change process.
Heat is a necessary part of the phase change process, as it increases the kinetic energy of particles and can cause them to enter different phases. As the temperature of a substance increases, the particles move faster and their kinetic energy increases. This increased energy can cause particles to break apart and enter a new phase, such as from a solid to a liquid or from a liquid to a gas.
3. Describe how pressure can affect the phase change process.
Pressure can also play an important role in the phase change process. Increasing the pressure on a substance can cause particles to move closer together or further apart, thus affecting their state. When the pressure is increased, the particles vibrate faster, which can cause them to enter a different phase. The higher the pressure, the more likely a particle is to move into a different phase.
4. Explain the implications of these effects on phase change in practical applications.
The effects of heat and pressure on phase change have a number of practical applications. For instance, pressure is often used to liquefy gases in order to make them easier to store and transport. In addition, heat and pressure can be used to create new materials with different properties. Heat and pressure can also be used to produce chemical reactions, such as in the production of plastic. Thus, understanding the effects of heat and pressure on phase change can be extremely beneficial in a variety of industrial contexts.
Applying Phase Change Worksheet Answers to Real-World Problems: Exploring How Phase Changes Can Be Used in Everyday Situations
Phase changes are a natural phenomena that can be observed in everyday life. The ability to recognize and apply the principles of phase change can be incredibly useful in a variety of situations. In this article, we will explore how phase changes can be applied to real-world problems and used to our advantage in everyday situations.
One of the most common uses of phase change is in the control of temperature. By controlling the phase of a substance, such as water, it is possible to adjust the temperature of an environment or object. For example, when ice is heated, it melts and changes from a solid to a liquid, which is a process known as melting. This process releases a large amount of heat energy, which can be used to cool down the surrounding environment. Similarly, when liquid water is cooled, it freezes and changes from a liquid to a solid, which is a process known as freezing. This process absorbs a large amount of heat energy, which can be used to warm up the surrounding environment.
Another use of phase change is in energy storage. By changing the phase of a substance, it is possible to store energy in a very efficient way. For example, water can be heated to form steam, which is a gas. This gas can be used to drive a turbine and generate electricity. When the electricity is no longer needed, the steam can be cooled back into liquid water and stored until it is needed again.
Finally, phase changes can also be used to purify and filter water. By freezing water, it is possible to remove impurities from the water by forcing them to remain in the solid portion of the ice. Similarly, by condensing steam, it is possible to remove impurities from the gas by forcing them to remain in the liquid portion of the condensate.
By understanding and applying the principles of phase change, it is possible to use them to solve many real-world problems. By controlling the temperature of our environment, storing energy in efficient ways, and purifying water, we can make the most of this natural phenomena and use it to our advantage in everyday situations.
Images of Phase Change Worksheet Answers
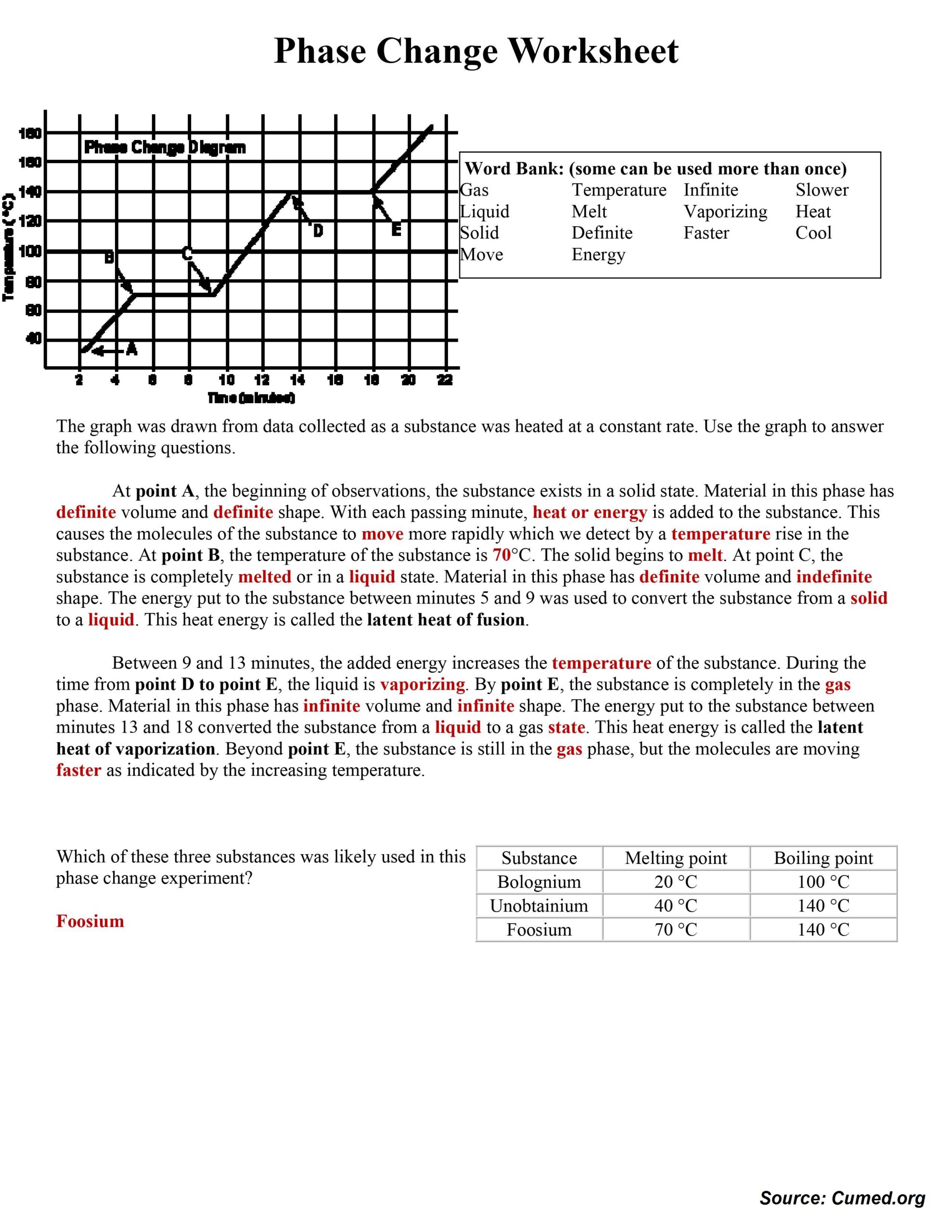
Download Phase Change Worksheet Answers
Download Phase Change Worksheet Answers: Click Here
Conclusion
In conclusion, the Phase Change Worksheet Answers provide a helpful way to understand and apply the concepts of phase changes and energy. By working through the questions on the worksheet, individuals are able to gain a better understanding of the various types of changes that can occur in matter and the associated energy transfer. This understanding can then be applied to real-world scenarios, enabling the user to make more informed decisions.
