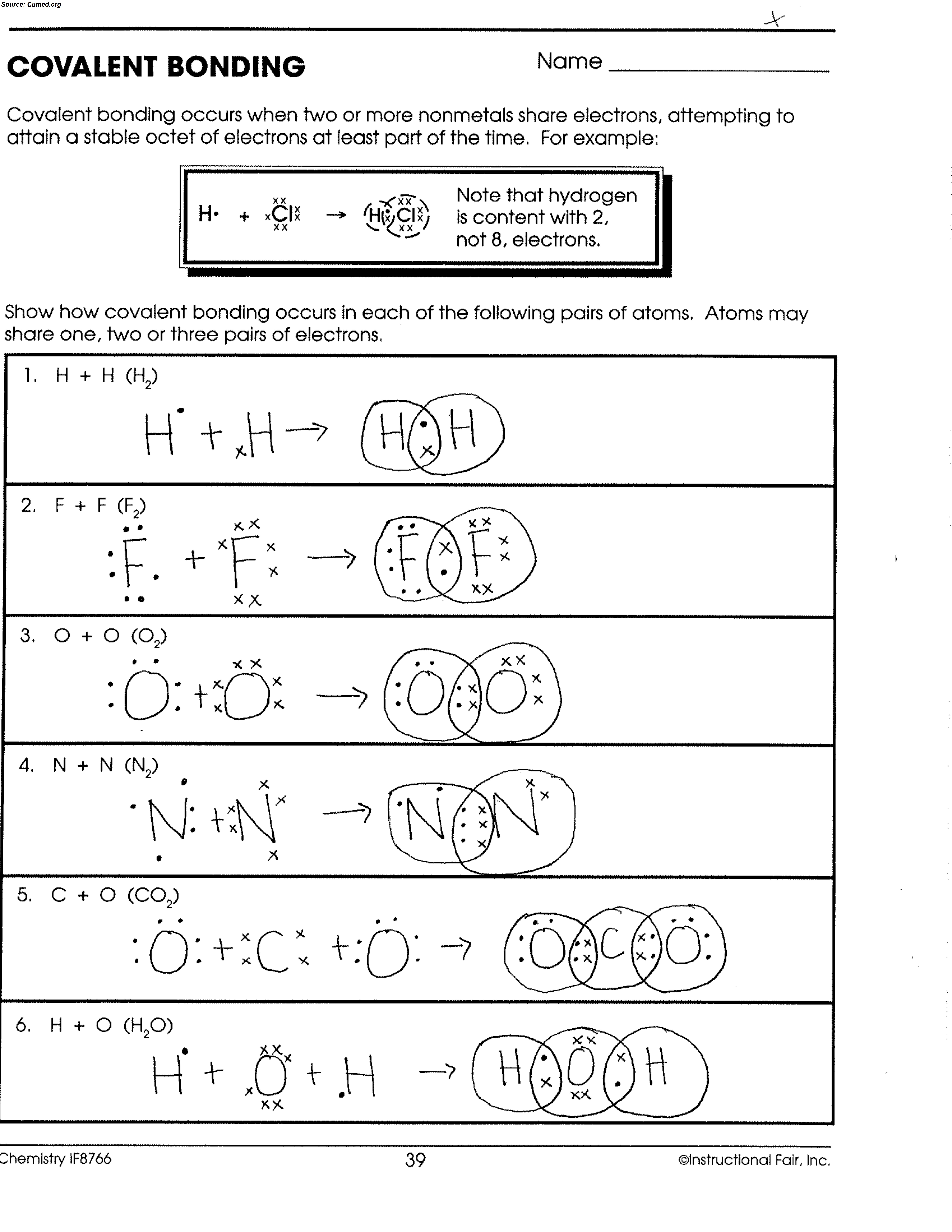
The Covalent Bonding Worksheet Answer Key is a great resource for those who need to understand the principles of covalent bonding. This worksheet provides an easy-to-follow explanation of how covalent bonds form between two atoms. It also provides an overview of the different types of covalent bonds and how they work. The answer key provides a clear understanding of the concepts and makes it easier to answer questions related to covalent bonding. With this resource, students can gain a better understanding of this important topic and apply it to their study of other chemistry topics.
Analyzing Valence Electrons and Covalent Bonding with a Covalent Bonding Worksheet Answer Key
The understanding of valence electrons and covalent bonding is essential for the study of chemistry. Valence electrons are those electrons in the outermost energy level of an atom that are available for chemical interaction. Covalent bonding is a type of chemical bonding where two atoms share electrons in order to attain a more stable electron configuration.
To better understand the concept of valence electrons and covalent bonding, a covalent bonding worksheet can be used. The worksheet may be used to identify the various types of covalent bonds, the number of valence electrons, and the electron configuration of each atom in the bond.
Contents
- 0.1 Analyzing Valence Electrons and Covalent Bonding with a Covalent Bonding Worksheet Answer Key
- 0.2 Exploring Molecular Geometry and Intermolecular Forces with a Covalent Bonding Worksheet Answer Key
- 0.3 Understanding the Different Types of Covalent Bonds with a Covalent Bonding Worksheet Answer Key
- 0.4 Utilizing Covalent Bonding Worksheet Answer Key to Facilitate Bonding in Organic Chemistry
- 0.5 Images of Covalent Bonding Worksheet Answer Key
- 0.6 Download Covalent Bonding Worksheet Answer Key
- 1 Conclusion
- 1.1 Some pictures about 'Covalent Bonding Worksheet Answer Key'
- 1.1.1 covalent bonding worksheet answer key
- 1.1.2 covalent bonding worksheet answer key pdf
- 1.1.3 covalent bonding activity worksheet answer key
- 1.1.4 covalent bonding review worksheet answer key
- 1.1.5 covalent bonding worksheet 1 answer key
- 1.1.6 collisions covalent bonding worksheet answer key
- 1.1.7 covalent bonding worksheet 3 answer key
- 1.1.8 covalent bonding practice worksheet answer key
- 1.1.9 chemthink covalent bonding worksheet answer key
- 1.1.10 covalent bonding webquest worksheet answer key
- 1.2 Related posts of "Covalent Bonding Worksheet Answer Key"
- 1.1 Some pictures about 'Covalent Bonding Worksheet Answer Key'
To begin the worksheet, the student should first determine the number of valence electrons of each atom in the bond. This can be done by counting the number of electrons in the outermost energy level of each atom. For example, if a carbon atom is bonded with a hydrogen atom, the carbon atom will have four valence electrons, and the hydrogen atom will have one valence electron.
Next, the student should identify the type of covalent bond formed by the two atoms. A single bond is formed when two atoms share one pair of electrons, a double bond is formed when two atoms share two pairs of electrons, and a triple bond is formed when two atoms share three pairs of electrons. After determining the type of bond, the student should then draw the Lewis structure of the molecule, which is a diagram showing the arrangement of the atoms and their valence electrons.
Finally, the student should calculate the formal charge of each atom in the molecule. This is done by subtracting the number of valence electrons from the number of atomic orbitals in the atom. A positive formal charge indicates an electron deficiency in the atom, and a negative formal charge indicates an electron surplus in the atom.
By completing a covalent bonding worksheet, students can develop a better understanding of valence electrons and covalent bonding. Through consistent practice, they can gain the knowledge and skills needed to accurately analyze the structure and properties of different molecules.
Exploring Molecular Geometry and Intermolecular Forces with a Covalent Bonding Worksheet Answer Key
Introduction:
Molecular geometry and intermolecular forces are key concepts in understanding the behavior of molecules. Molecular geometry is the arrangement of atoms in a molecule and determines its shape, while intermolecular forces are the forces that exist between molecules. This covalent bonding worksheet will allow students to explore the influence of molecular geometry and intermolecular forces on the physical and chemical properties of molecules.
Part 1: Molecular Geometry
1. What is molecular geometry?
Molecular geometry is the arrangement of atoms in a molecule and determines its shape. It is determined by the number of valence electrons in the molecule and the type of bonds between them. Molecular geometry affects the physical and chemical properties of the molecule, including its boiling point, melting point, and other physical properties.
Understanding the Different Types of Covalent Bonds with a Covalent Bonding Worksheet Answer Key
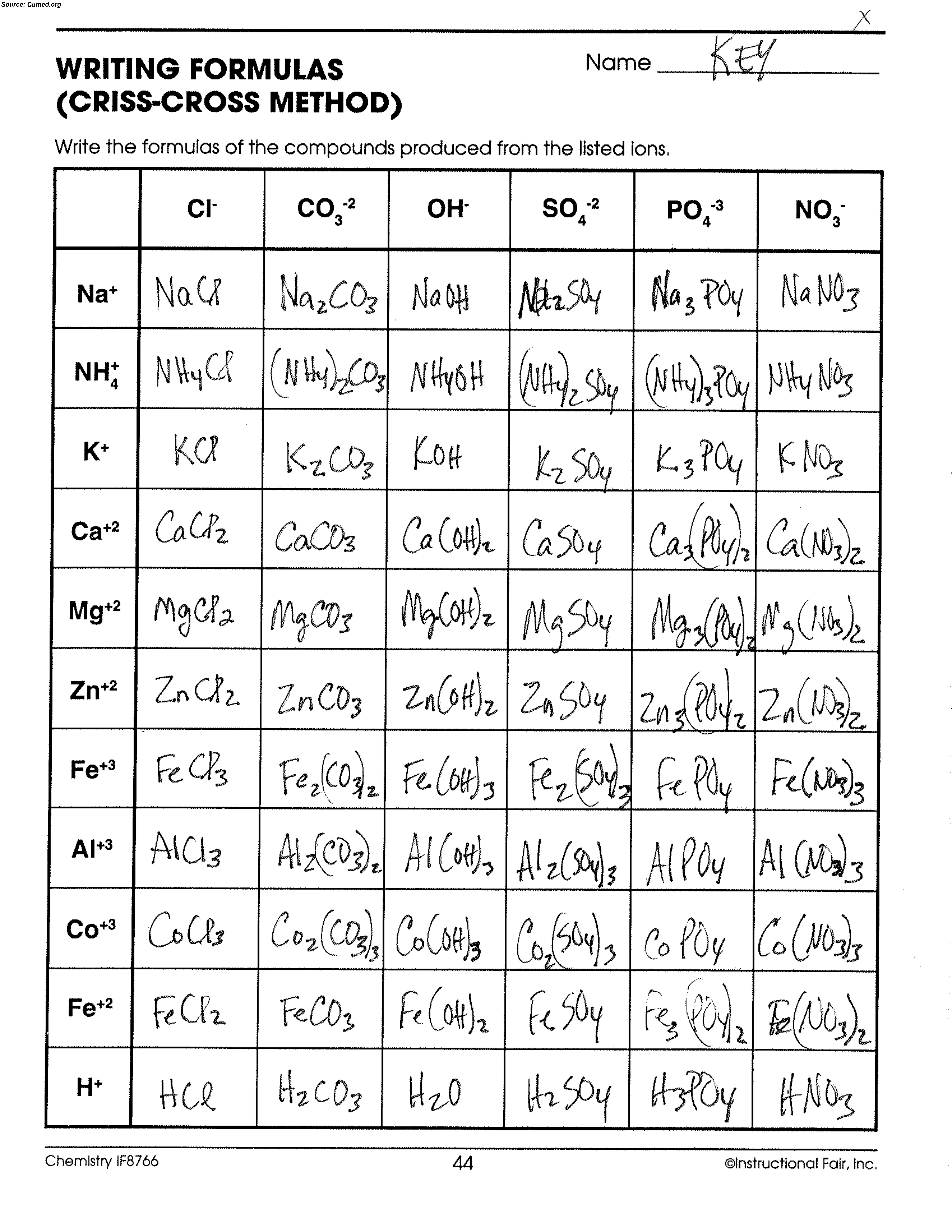
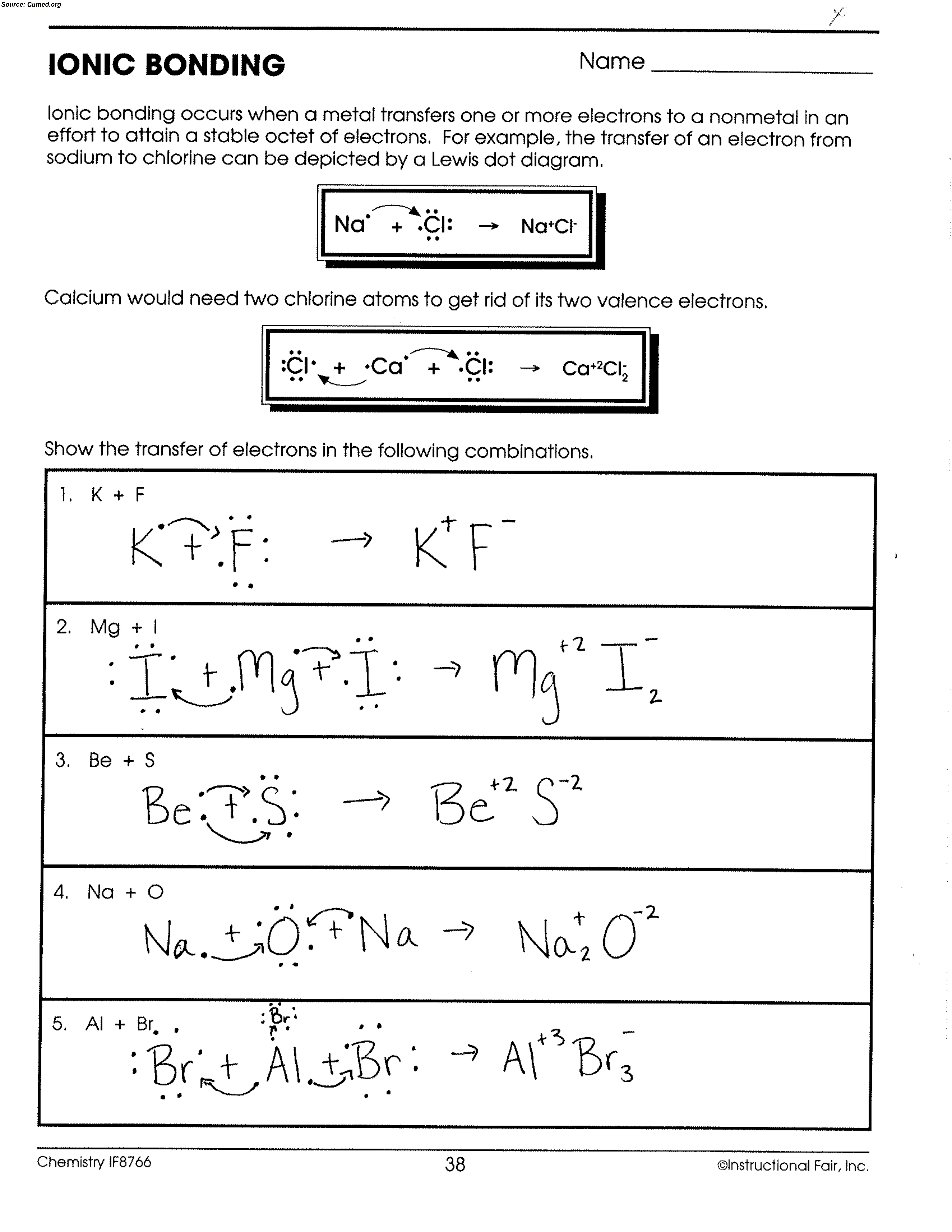
Covalent bonds are a type of chemical bond formed when two atoms share electrons. These bonds are formed between two nonmetals, and the atoms involved in the bond are held together by the attraction between the shared electrons. Understanding covalent bonds is important for understanding how different molecules are formed and how they interact with one another.
A covalent bonding worksheet answer key can be a useful tool for understanding the different types of covalent bonds and how they are formed. The worksheet typically includes questions about the types of covalent bonds, their bond energies, and the ways in which covalent bonds can be broken.
The most common type of covalent bond is a single covalent bond. This bond is formed when two nonmetals share a pair of electrons. Single covalent bonds are typically the strongest type of bond, and they are usually the most stable.
Double covalent bonds are formed when two nonmetals share two pairs of electrons. These bonds are usually weaker than single covalent bonds, but they can still be quite strong. Triple covalent bonds are also possible, in which three pairs of electrons are shared between two nonmetals. Triple covalent bonds are usually the weakest type of bond, but they are still very strong.
The bond energies for the different types of covalent bonds can vary depending on the type of atoms involved in the bond. Generally, single bonds have higher bond energies than double or triple covalent bonds. Bond energies can also vary depending on the size of the atoms involved, with larger atoms having higher bond energies than smaller atoms.
Covalent bonds can be broken in a variety of ways. One way is through chemical reactions, where the bond is broken when a chemical reaction takes place. Another way is through physical force, where the bond is broken when two atoms are pulled apart. The type of bond involved will determine the amount of force needed to break the bond.
A covalent bonding worksheet answer key can be a useful tool for understanding the different types of covalent bonds and how they are formed. With this information, students can gain a better understanding of how different molecules are formed and how they interact with one another.
Utilizing Covalent Bonding Worksheet Answer Key to Facilitate Bonding in Organic Chemistry
Organic chemistry is an important field in the sciences, and covalent bonding is an essential concept to understand. Covalent bonding involves the sharing of electrons between two atoms, and can be used to create the various molecules and compounds found in organic chemistry. To facilitate the learning of covalent bonding and its applications in organic chemistry, this worksheet is designed to provide a comprehensive overview of the topics related to covalent bonding.
This worksheet begins by introducing the different types of covalent bonds and their respective properties. It then moves on to discuss the different structures of molecules and their corresponding bonding patterns. Finally, the worksheet provides examples of how covalent bonding is used in organic chemistry.
The worksheet includes a variety of questions and exercises related to covalent bonding. These include questions about types of bonds, bond lengths and strengths, and the different types of molecules formed by covalent bonding. There are also several diagrams to help students visualize the concept of covalent bonding.
In addition to the questions and diagrams, this worksheet also includes an answer key to help students understand the material and practice the concepts. This answer key includes step-by-step solutions to the questions, detailed explanations of the concepts, and examples of how covalent bonding is applied in organic chemistry.
The answer key is designed to be used in conjunction with the worksheet. It should be used to help students check their work and understand the material in more depth. It can also be used to teach students how to apply covalent bonding in their own research projects.
This Covalent Bonding Worksheet and Answer Key is an excellent resource for students studying organic chemistry. It provides an overview of the concepts related to covalent bonding and can help students understand the material and apply it in their own research. The answer key can also be used to facilitate the learning process by providing step-by-step solutions and detailed explanations of the concepts.
Images of Covalent Bonding Worksheet Answer Key


Download Covalent Bonding Worksheet Answer Key
Download Covalent Bonding Worksheet Answer Key: click here
Conclusion
The Covalent Bonding Worksheet Answer Key provides a comprehensive overview of the different types of covalent bonds and their properties. This answer key can help students better understand the different types of covalent bonds and the effects they can have on a molecule. Understanding these properties can help students better understand the behavior of molecules and their interactions with each other. With this knowledge, students can better predict the behavior of molecules and design better experiments.
